

|
Figure 3 |


|
Chemical reactions, parameter definitions and thermodynamic constraints
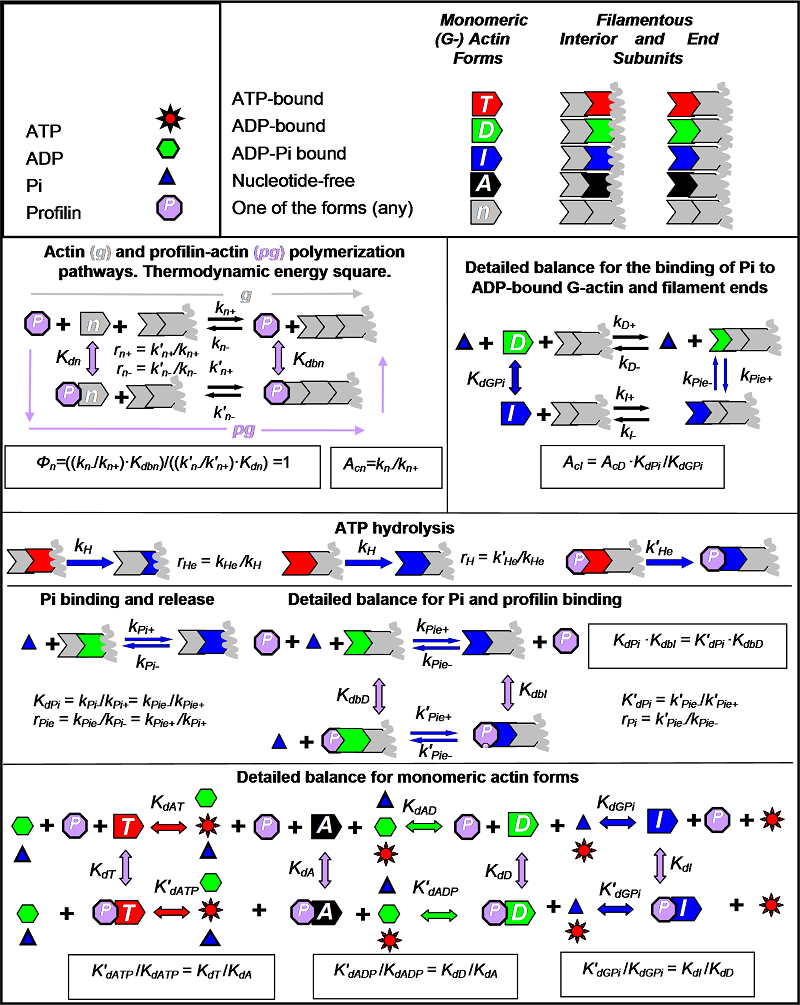
The model considers a steady state copolymerization of four monomeric actin forms (shown as pentagons): ATP-(T), ADP-(D), ADP and Pi-bound (I), and nucleotide-free (A). T is shown in red, D in green, I in blue, and A in black. Actin subunits that could have any type the bound nucleotide are shown in gray. Filamentous actin subunits are represented by chevrons with the colors corresponding to the bound nucleotides. Filaments are depicted as two or more chevrons with a filament end opposite to the open side of a chevron. Distance from the filament end is measured in terms of i, the number of subunits, so that i = 0 corresponds to monomeric actin, and i = 1 always corresponds to the subunit at the filament end.
Profilin (purple octagon) binds to monomeric actin subunits and to the filament ends of each nucleotide type. Each nucleotide form of monomeric actin and their complexes with profilin (PT, PD, PI, and PA) bind to the filament ends. Other reactions include: binding of ATP (red sun) or ADP (green hexagon) to A (or PA) to form T or D (or PT or PD), binding of inorganic phosphate Pi (blue triangle) to D (or PD), or the ADP-bound F-actin subunits (or the profilin-capped D-type filament ends) to form I or the ADP-Pi bound F-actin form (or the profilin-capped I-type filament ends), and ATP hydrolysis by ATP-bound filamentous actin subunits, which is the only irreversible reaction. All other reactions are reversible. Thermodynamic constraints are shown in boxes. Parameter definitions are shown next to reactions (without boxes).