

|
Figure 2 |


|
Thermodynamic energy square
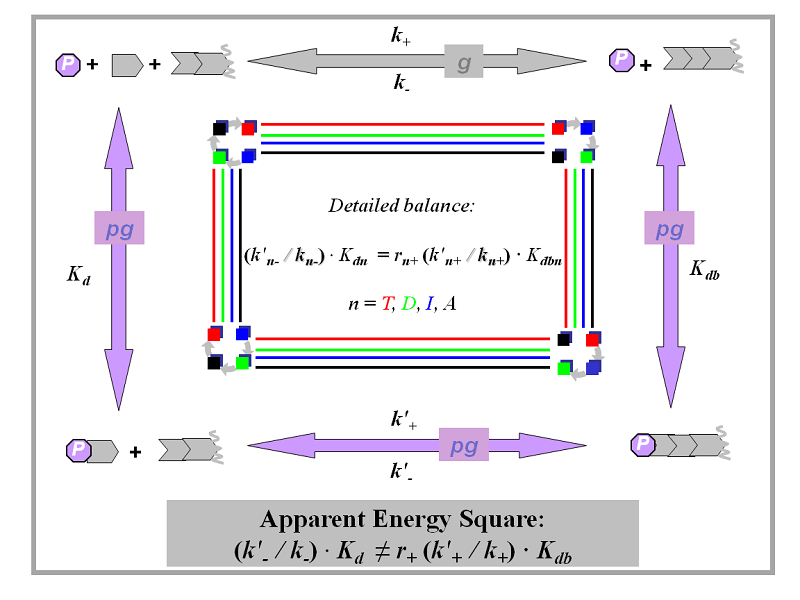
Two pathways of actin filament elongation in presence of profilin
Monomeric actin subunits are shown as gray pentagons, profilin is shown as purple octagon, F-actin subunits are represented by chevrons and filaments are depicted as two or more chevrons with a filament end opposite to the open side of a chevron.
In presence of profilin, there are two possible pathways for actin filament elongation. First pathway for elongation of actin filaments (g, shown with grey arrows) is direct elongation through binding of monomeric actin subunits (grey pentagons) to the barbed end with the rate k+ to obtain a filament one subunit longer. The second pathway (pg, shown with purple arrows) is elongation through binding of profilin (P, purple octagon) to monomeric actin, formation of profilin-actin complex (with the equilibrium dissociation constant Kd), then binding of the profilin-actin complex with the rate k'+ to the barbed end with subsequent dissociation of profilin (with the equilibrium dissociation constant Kdb). Reverse process for the pathway pg involves association of profilin with the barbed end, dissociation of the profilin-actin complex with the rate k'-, and then dissociation of profilin from monomeric actin. The reverse process for the pathway g is simply a dissociation of the actin subunits from the filament end with the rate k-.
The thermodynamic “energy square” shown here is one of the thermodynamic constraints (or detailed balance requirements) that follow from the second law of thermodynamics. They relate rate and equilibrium constants for each pure form n, where n = T, D, I, or A. Only an “apparent” (grey) energy square that relates apparent constants and does not distinguish between different nucleotide identities of actin subunits is allowed to be misbalanced. Each energy square that is limited to a particular form of subunit n (four colored squares) must necessarily be balanced. The subscript n denotes the four actin subunits forms: ATP-, ADP-, ADP-Pi-bound, and nucleotide-free.